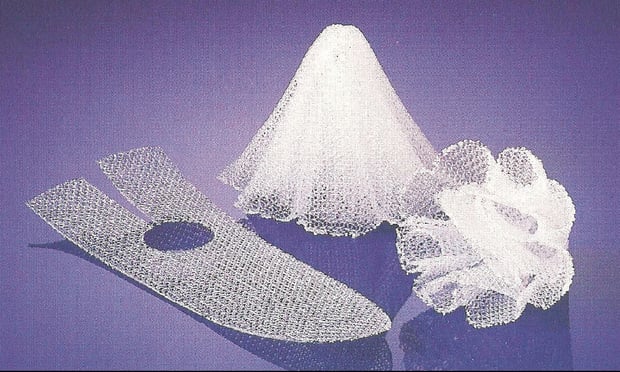
A Johnson & Johnson subsidiary has lost a bid to upend an $11.1 million verdict in the first bellwether trial over allegedly harmful pelvic mesh implants.
Atlantic County Superior Court Judge Carol Higbee on Tuesday denied motions by Ethicon for a new trial and judgment notwithstanding the verdict that would have struck a jury award of $3.35 million in compensatory damages and $7.76 million in punitive damages from early last year.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]