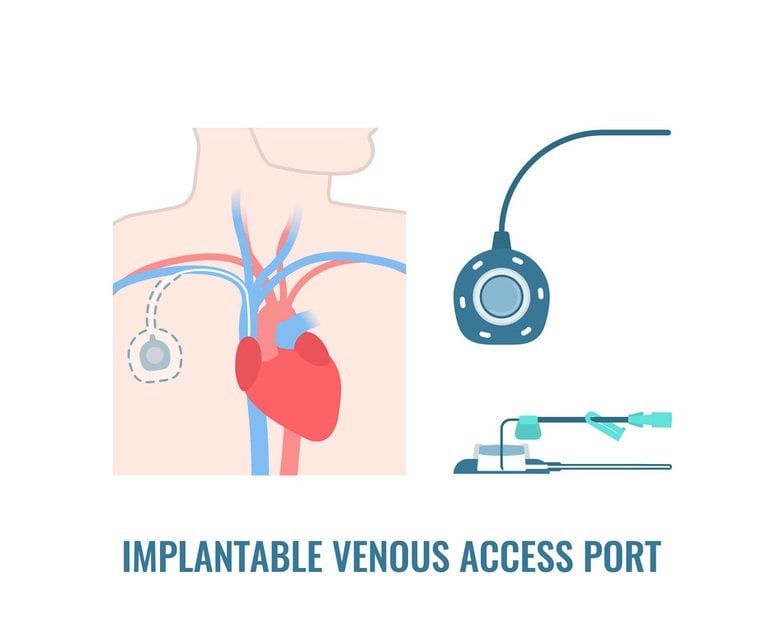
In 2019, the U.S. Food and Drug Administration discontinued a reporting program that for years collected confidential data from medical device manufacturers about problems with their products.
When that data later became public, lawyers uncovered a big issue with C.R. Bard Inc.’s PowerPort, a medical device, which is implanted under the skin to provide intravenous chemotherapy, nutrition and other fluids.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]