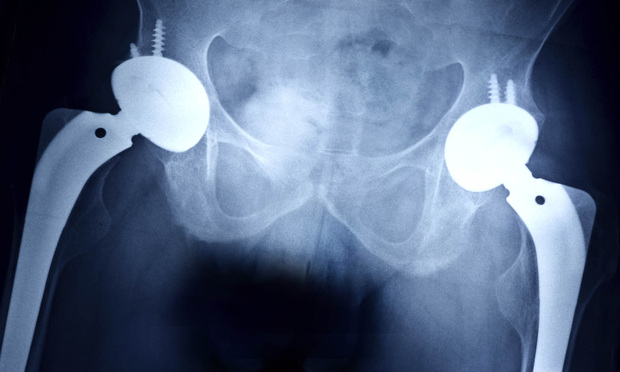
Thousands of plaintiffs in New Jersey and around the country who had surgery to remove failed hip implants settled their claims Nov. 3 in a deal that is expected to pay out more than $1 billion.
The global settlement with Howmedica Osteonics Corporation, a Mahwah, N.J., corporation that does business as Stryker Orthopaedics, resolves the claims of about 3,000 individuals who were implanted with Stryker Rejuvenate and ABG II Modular hip stems.
This content has been archived. It is available through our partners, LexisNexis® and Bloomberg Law.
To view this content, please continue to their sites.
Not a Lexis Subscriber?
Subscribe Now
Not a Bloomberg Law Subscriber?
Subscribe Now
LexisNexis® and Bloomberg Law are third party online distributors of the broad collection of current and archived versions of ALM's legal news publications. LexisNexis® and Bloomberg Law customers are able to access and use ALM's content, including content from the National Law Journal, The American Lawyer, Legaltech News, The New York Law Journal, and Corporate Counsel, as well as other sources of legal information.
For questions call 1-877-256-2472 or contact us at [email protected]