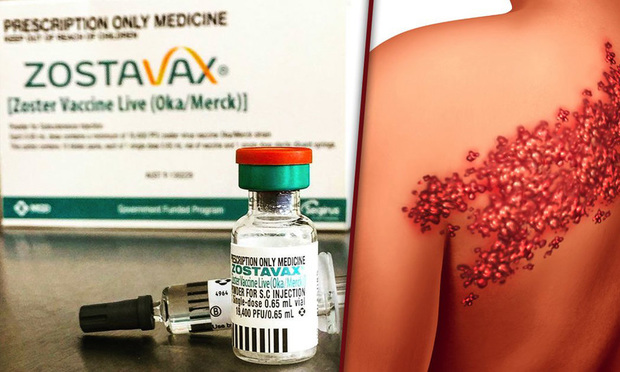
A federal judicial panel has sent about 100 lawsuits brought over Merck’s shingles vaccine to Pennsylvania.
Thursday’s order by the U.S. Judicial Panel on Multidistrict Litigation sends all federal cases brought over Zostavax to U.S. District Judge Harvey Bartle of the Eastern District of Pennsylvania, who has been overseeing the first case since 2016. The lawsuits, filed in Pennsylvania, New Jersey, New York, Wisconsin and Massachusetts, allege that Merck failed to warn that the virus in the vaccine caused shingles, brain damage and death, among other things.